Mosie Baby's $129 DIY Insemination Kit Receives FDA Approval
Mosie Baby makes home insemination an accessible and viable choice for those seeking to conceive.
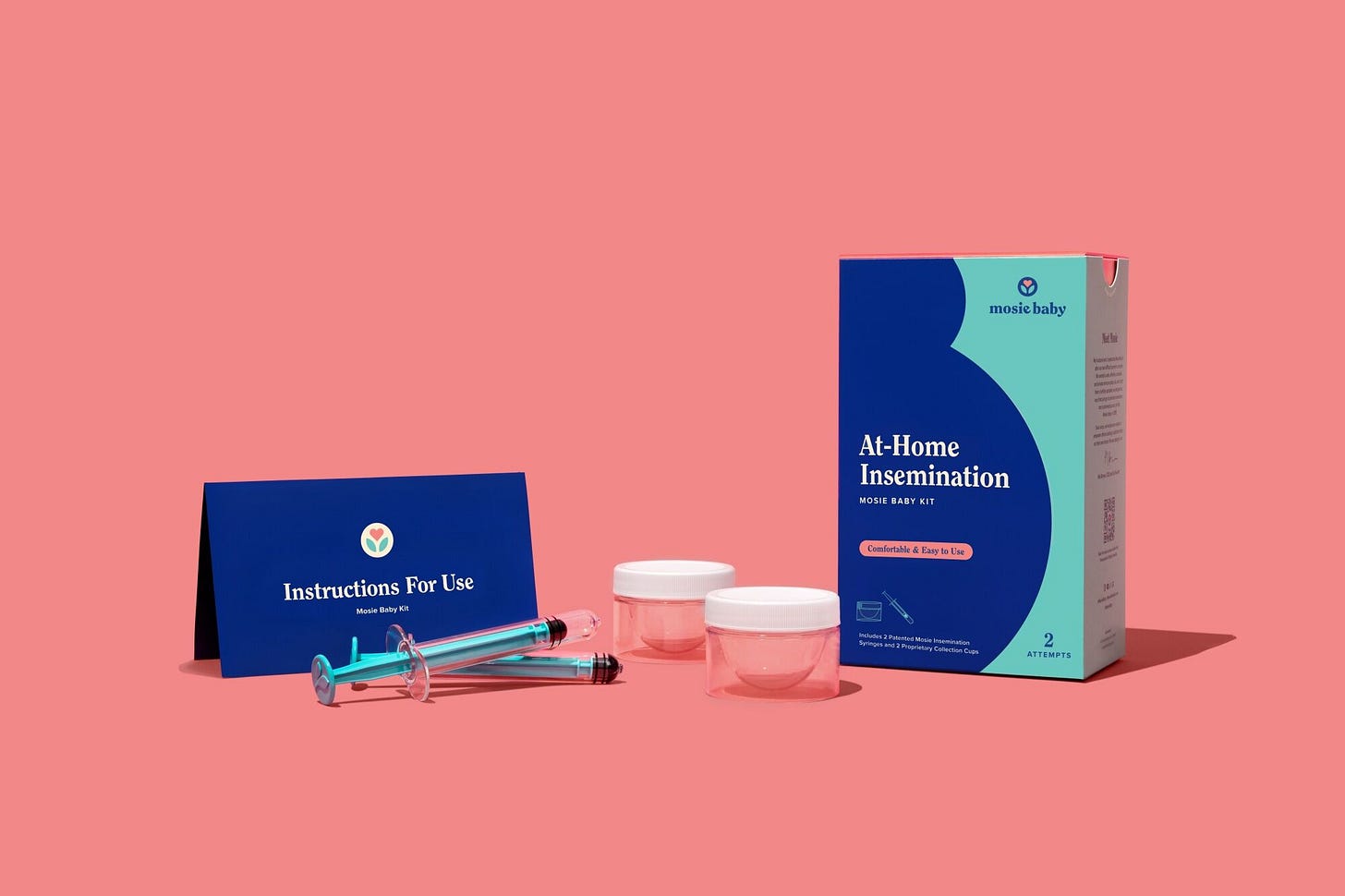
In a significant development for the fertility industry, Mosie Baby has gained FDA approval for its DIY Artificial Insemination Kit, priced at $129. The Austin-based company, founded in 2015, aims to provide an accessible and cost-effective alternative for couples struggling with conception, positioning itself as the first at-home kit cleared by the FDA for intravaginal insemination. The FDA's stamp of approval not only signifies regulatory compliance but also underscores the kit's rigorous testing, instilling confidence in both end-users and the medical community regarding its high manufacturing and performance standards.
The Mosie Baby kit distinguishes itself from traditional intrauterine insemination (IUI) procedures by facilitating sperm insertion into the vagina through a syringe, offering a less invasive and more intimate approach akin to natural conception. Co-founders Maureen and Marc Brown, drawing from personal experiences with IUI, sought to create a more comfortable option for individuals using donor sperm, struggling with intimacy, or seeking an alternative before pursuing more complex fertility procedures. While acknowledging the importance of seeking medical advice, Mosie Baby's kit, available at CVS stores and major online platforms, is positioned as a first-line treatment for those navigating the challenges of conception, supported by the recent $10 million investment that propels the company towards further expansion and innovation in 2024.
Read more from Maureen Brown in Founders Everywhere.